Graphene features a number of unique properties such as electrical conductivity, thermal conductivity, light transmittance and excellent mechanical properties. Graphene can also be considered as the world’s thinnest and hardest nanomaterials in the present; it has broad application prospects closely related to human life in the fields of next-generation transistors, transparent conductive films, energy storage, chemical sensors, and functional composite materials.

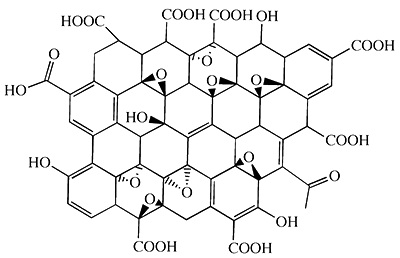

The hexagonal benzene ring of graphene surface features a high chemical stability, leading to weak interaction with other media. On the other hand, the strong van der Waals force between the sheet layers is resulted in neither hydrophilic nor lipophilic. In this case, graphene is hardly compatible with other media or polymer matrix, making it easy to aggregates.
To overcome the inherent shortcomings of graphene, surface modification treatment must be done. In other words, surface functionalization can improve its processability and compatibility, so as to increase and expand the range of practical applications. Modification by introduction of diverse functional groups enhances interfacial binding between the graphene and media or polymers. Since graphene oxide (GO) contains a wide range of reactive oxygen-containing functional groups, the use of these functional groups may react with other chemical molecules for the achievement of graphene modifications. Therefore, GO plays an essential role in terms of functional graphene.
The modified graphene can be better uniformly dispersed in the polymer matrix or medium, so that it can be easily processed and dispersed at functional graphene-filled polymer or plastic compounds. The performance of the formed functional composite materials (such as polymer composites, ceramic composite materials etc) can be greatly improved. If GO is employed as the precursor, its surface-activated reactive groups are able to be used to process the chemical modification of the surface. Then reduction of functionalized GO to various modified graphene is called graphene derivatives.
The properties of composite materials (such as electrical conductivity, thermal conductivity, mechanical strength, etc) are mainly influenced by the additive properties, type, dosage and interfacial compatibility between additives and substrates. If a wide variety of additives (such as graphene nanoplatelets or graphene that is not reduced graphene oxide) are used in composites (such as polymer composites, ceramic composites, metal composite materials, etc.), the poor dispersion in the graphene-filled polymer or solution can be found. This interfacial incompatibility leading to the generation of agglomeration will produce a considerable influence on the properties of the resultant material. These characteristics limit the possibility of potential application of graphene nanoplatelets or graphene, not rGO.
To improve interfacial compatibility between the components of composite material and to solve the poor bonding strength between phase and phase, the composite components, especially for additives, need to be possibly modified. This will improve the interfacial properties of the composites and thereby enhance the performance of the composites.
GO (i.e., graphene oxide) is a readily soluble derivative of graphene. Since GO both contains a variety of oxygen-containing functional groups (e.g. a carboxyl group, a hydroxyl group, an epoxy group, a carbonyl group, etc.) and possess the amphiphilic property which has both lipophilic and hydrophilic groups in the structure analysis, various stable composite slurries with good dispersion conditions can be readily attained. This makes the properties (electrical conductivity, thermal conductivity, mechanical strength, etc.) of functional composites (polymer composites, ceramic composites, metal composites, etc.) able to demonstrate completely.

Graphene discovered in the laboratory in 2004, at that time two British scientists used Scotch Tape to strip thin flakes of graphite away from the graphite surface. By repeating this process, finally, they got a structure of a single-layered sheet with 2D nanomaterials composed of just one layer of carbon atoms, which is called graphene. It is generally recognized that entering the field on industrial scale production for graphene is not far away. Therefore, two scientists were awarded the 2010 Nobel Prize in physics.
Graphene is the perfect two-dimensional structure. After decomposition, its structure can be turned into zero-dimensional fullerenes. As rolled into a cylinder, it can form nanotubes. When stacked together, it can form a 3D graphite. Consequently, graphene is the basic unit of the graphite material (i.e. carbon allotropes).
Graphene is the thinnest and the hardest material in the world. If graphene material is employed to replace plastic packaging material for food, the graphene food packaging bag with 100 nm thickness will be able to withstand the pressure of about 2 tons heavy items, and will not break. It can be applied in ultralight body armor, thin and ultralight aircraft material, light vehicles, and it could eventually result in the manufacture of space elevator rising tens of thousands of miles into space.
Graphene is the world’s best conductive material, the electron velocity in graphene reaches 1/300 of the speed of light, far exceeding the velocity of electrons in the normal conductor. Consequently, it is expected to be employed instead of silicon, which would become thinnest next-generation electronic components or transistors with faster conduction speed.
Graphene is the perfect two-dimensional structure. After decomposition, its structure can be turned into zero-dimensional fullerenes. As rolled into a cylinder, it can form nanotubes. When stacked together, it can form a 3D graphite. Consequently, graphene is the basic unit of the graphite material (i.e. carbon allotropes).
Graphene has high conductivity, optical transparency, and mechanical toughness (e.g. graphene can be stretched 20% of its length without any damage). Graphene can replace the ITO as the transparent conductive material (i.e. flexible electrode), and it can be applied to the touch panel, solar cell with a transparent conductive film and OLED panel.
Graphene (i.e. rGO) is the preferred material for the preparation of high-performance rechargeable battery electrodes. It can be used as positive and negative battery materials. Graphene employed as a lithium-ion battery conductive additive, can significantly improve the large current charging and discharging performance of battery and cycle stability and security. Graphene itself possessing ultra-high specific surface area and diversely hollow pore structure is capable of dramatically increasing supercapacitor energy density and power density, which help dramatically enhance the characteristics of the supercapacitor.
Scientists found that graphene (i.e. rGO) and graphene oxide (GO) were expected to be applied in many biotechnology or biopharmaceutics in the future. Since GO that is harmless to humans can promote the growth of bacteria and mammalian cells, it can participate in cultured human cells in tissue engineering or increase biopharmaceutical production. Functionalized GO also has excellent biocompatibility and stability.
Four main methods for preparation of graphene include micromechanical stripping method, epitaxial growth method, chemical vapor deposition (CVD) method and graphite oxide reduction method. Graphite oxide reduction was used to prepare graphene materials for us. When natural graphite was reacted with strong acids in the presence of oxidants, it converted into graphite oxide. Monolayer or few layer graphene oxide (GO) was prepared by ultrasonic stripping dispersion. Then through the heat treatment or adding a reducing agent to remove oxygen-containing functional groups of GO surface, graphene can be obtained.



Polymer composite materials
Features / Superior electrical & thermal conductivity, Excellent mechanical & barrier property, Thermal stability, Light transmittance
Graphene can be utilized as a functional additive in numerous resin and plastic substrates such as epoxy, phenol resin, PS, PVA, PET, PMMA, PP, PU, PI to develop various composite materials of electrical and thermal conductivity, heat resistance, gas barrier and structural reinforcement. Also, graphene can be made into PVA and PET films due to its high transmittance.

Paint/Ink
Features/ Superior electrical & thermal conductivity, Corrosion resistance
Graphene can be utilized as a functional additive to develop novel coatings/paints and inks for different applications, such as heavy antiseptic paint, electromagnetic shielding paint, thermal coating used for LED lamps, conductive ink used for printed circuit boards, etc.

Lithium ion batteries
Features / Large surface area, Excellent electrical conductivity
Graphene can be added to electrode materials of L-ion batteries to improve cycle life, stability, capacity and achieve a rapid charge-discharge performance.

Solar cell
Features / High carrier mobility, Light transmittance
Graphene is able to replace Si or ITO electrode as the transparent conductive film due to its high transmittance and low impedance.

Super-capacitors
Features / Large surface area, Excellent electrical conductivity
Graphene can be applied to electrode (coated aluminum foil) of super-capacitors to improve energy density, capacity and cycle life.

Catalyst
Features / Large sucface area, High carrier mobility
Graphene oxide can be functionalized (introduction of a carboxyl group, a carbonyl group, an epoxy group, etc.) to constitute controllable chemical defects, which are able to be nucleation centers that metals grow to control the growth of metals for improving their catalytic properties.

Biosensor
Features / high carrier mobility
Graphene oxide can be used for selective detection of DNA, gene and protein.

Humidity sensor
Features / hydrophilic
Humidity sensors manufactured by graphene oxide can increase the sensitivity and linear range.

Biomedical
Features / large surface area, biological comparability, Chemical stability, thinness,strength, Permeability, hydrophilic
Graphene oxide acting as a nano drug carrier is able to achieve high drug-load performance, targeting property and lower cytotoxicity, which helps cancer treatment. Auto-fluorescence of graphene oxide can also be used for live cell imaging of near-infrared light.

Antibacterial material
Features / antimicrobial activity, Slight cytotoxicity
Graphene oxide is able to inhibit the growth of Escherichia coli and increase antibacterial ability of composite materials combined with chitosan.

Thermal dissipation material
Features / high thermal conductivity
Graphene can be made of fins or cooling films applied to thermal management of smart phone, laptop and LED.

Electronic devices
Features / high carier mobility, Electrical conductivity, Chemical stability
Graphene helps reduce switching time of devices and achieve operating responses of high frequency.